 I was taking a little break once I got home today, watching Xiaolin showdown - as is my pattern in the afternoons - and got a news break crawling across the bottom of the screen. I came in on the middle of the announcement, seeing words like evacuation and looting and explosion. First response in my head was a simple what the?
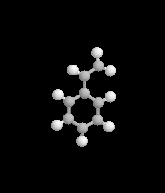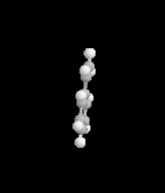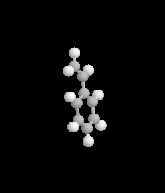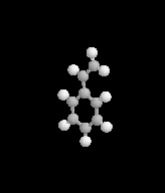
I was taking a little break once I got home today, watching Xiaolin showdown - as is my pattern in the afternoons - and got a news break crawling across the bottom of the screen. I came in on the middle of the announcement, seeing words like evacuation and looting and explosion. First response in my head was a simple what the?Apparently, a railcar full of styrene had begun to leak - in a big way, I guess - and has caused a decently large area of the east side of town to be evacuated with evacuations possibly staying through tomorrow. The mayor of Cincinnati has declared a state of emergency causing a curfew of 8pm tonight throughout that area. The Ohio River in the area nearest the train car has been closed to traffic, and the officials are reporting that there's a risk of explosion because the styrene's stabalizing agent expired months ago, leading to a build up of pressure and - according to reports - an increase in temperature which caused the pressure release valve to open wide.
I've got the tv on right now and am listening to a fire official saying that their explosive worry is that there could be an ignition source nearby which would cause a spark that would lead back to the tanker car - where they can't guarantee the structural intensity of the car.
I've got so many questions...
- It's my understanding that a liquid changing to a gas leads to a drop in temperature. How is this build up of temperature occuring?
- The supposedly dangerous level of styrene (according to the news) is 20 ppm, but the concentration here is only 0.5 ppm. How far away from the tanker does it drop below 20 ppm?
- The fire department says they've got people no closer than .5 miles. How are they dumping water on the tank without getting any closer?
- What is the styrene changing into as it becomes unstable?
- Is that compound inherently explosive?
- How can I use this information in my chemistry class?
- How massive an explosion could they expect to evacuate the are they're evacuated?
- Whose styrene is it?
- Mayor Luken actually said that he'd spoken to the chemical company and that they'd just said "hummina hummina." Seriously, who says "hummina hummina" to the mayor?
Seriously freaky stuff...very odd...

No comments:
Post a Comment